BLISTER PACK VISUAL QUALITY INSPECTION
DeepInspect®- FDA 21 CFR Part 11 Blister Pack Quality Inspection Software
DeepInspect® is an AI-powered blister pack inspection software designed for pharmaceutical manufacturers seeking 100% automated visual quality control. It accurately detects all critical defects, including missing tablets, double products, damaged capsules, empty pockets, foil seal defects, misaligned print, incorrect batch codes, color mismatches, and micro scratches. Its computer vision and deep learning algorithms enable inline inspection at high speeds, ensuring regulatory compliance, reduced recalls, and improved production yield.
DeepInspect® supports automated blister pack inspection, AI visual inspection for blister packaging, and machine vision for pharma blister inspection, with proven results in blister defect detection and foil misalignment correction. The software is fully FDA 21 CFR Part 11 compliant, supporting audit trails and electronic signatures.
DeepInspect® helps ensure blister packaging meets quality standards while minimizing false positives, reducing inspection costs, and ensuring zero-defect manufacturing.

APPLICATION

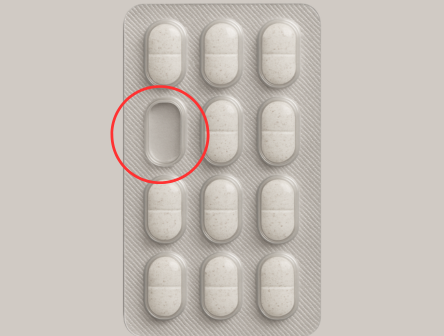
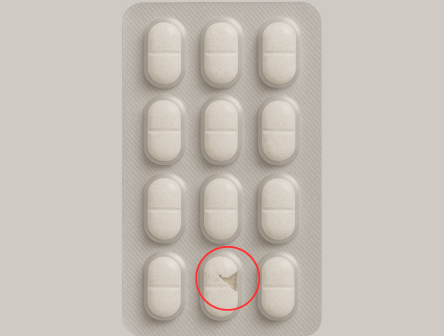
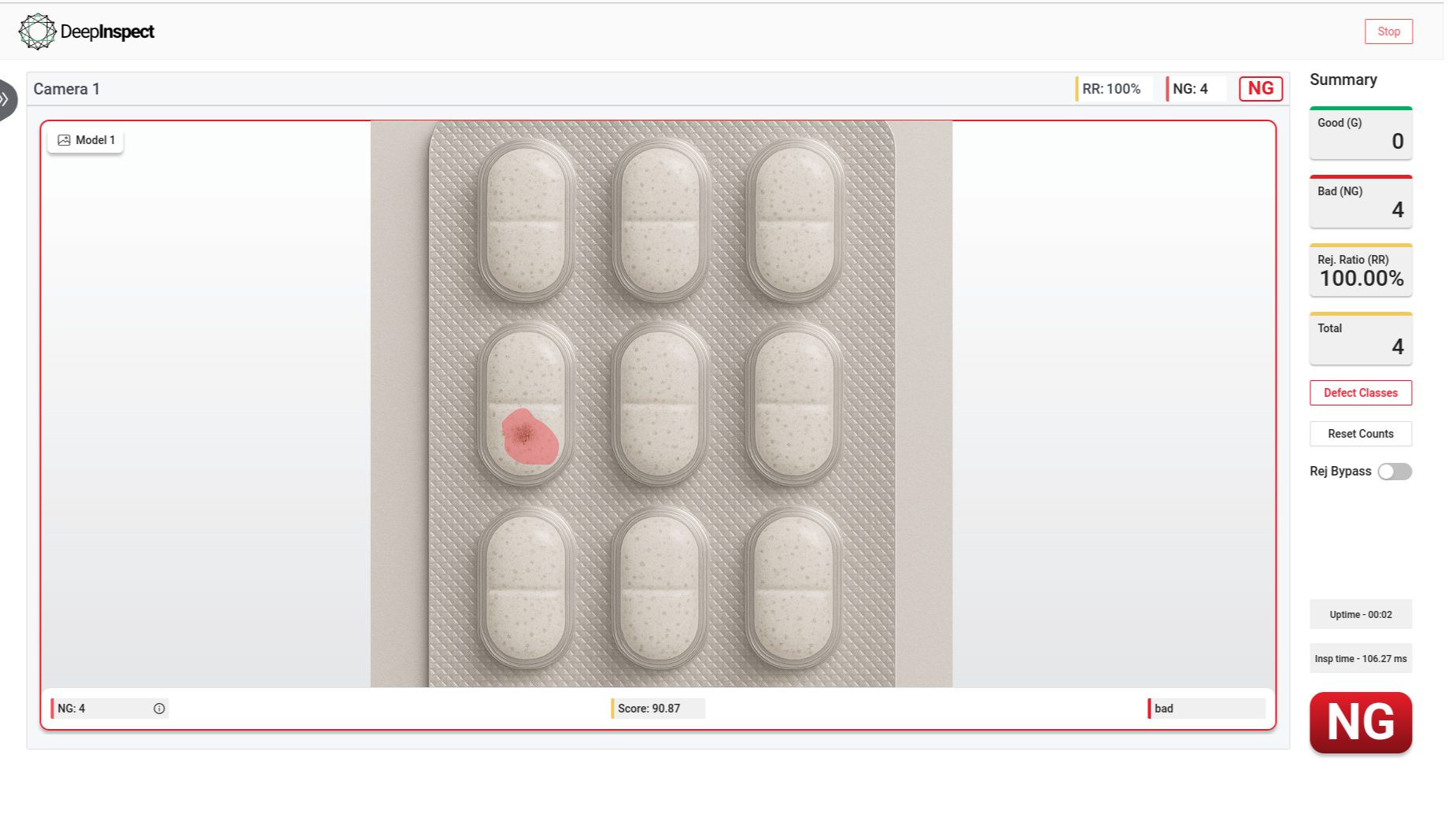
Identifies empty pockets, double fills, broken tablets, misalignment, and color mismatches.

Spots scratches, scuff marks, discoloration, air bubbles, wrinkles, and fading

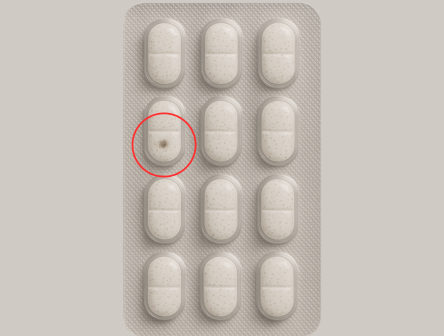
Detects foreign particles, dust, hair, stains, oil marks, and residues

Identifies deformed blisters, cracked plastic, edge chipping, warping, dents, and bulges
Impact
DeepInspect® revolutionizes pharmaceutical packaging vision inspection. It offers 99.5% defect detection accuracy
Computer vision blister pack inspection with 99.5% Accuracy
Empty Blister
Contaminants
Broken Tablets
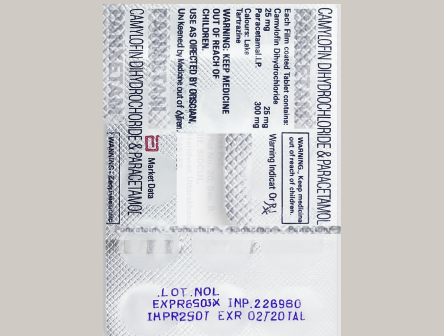
OCV/OCR
DeepInspect® - Best Vision AI Software for Blister Pack Quality Inspection
Best AI Tool for Blister Packaging Quality Control
High-Speed AOI System for Blister Pack Inspection

Why DeepInspect®
99.5% Inspection Accuracy: DeepInspect® delivers consistent, high-speed inspection accuracy of 99.5%, setting new benchmarks for reliability in automated quality control
Less Than 200 Good Images for Model Training: DeepInspect® requires fewer than 200 good images to learn, adapt, and begin accurate inspection with minimal setup effort
Model Training in Under 45 Minutes: DeepInspect® enables model creation and fine-tuning in under 45 minutes, reducing downtime and maximizing productivity
Inline blister pack inspection system for zero-defect pharma manufacturing

Our Customers
Trusted by the world’s leading manufacturers to power zero-defect production
Interested in Streamlining Blister Pack Inspection Process?
Book a free demo!
Inquiry Form
AI-powered blister pack inspection uses computer vision and deep learning algorithms to automatically detect defects such as missing tablets, damaged capsules, foil seal defects, and misaligned prints in pharmaceutical packaging. Systems like DeepInspect® ensure 99.5% accuracy in blister defect detection, reducing recalls and ensuring GMP compliance.
Automated blister pack inspection systems detect defects such as empty cavities, double fills, broken or chipped tablets, seal integrity issues, discoloration, wrinkles in foil, dust particles, and incorrect or missing batch codes
Yes, leading AI blister pack visual inspection software, DeepInspect®, is FDA 21 CFR Part 11 compliant.
DeepInspect detects even the minute surface defects like blister cavity defects, sealing & foil defects, contamination defects, printing defects, etc.
We support Area Scan, Line Scan, and Thermal cameras. Our software is compatible with industry-standard vendors like Basler, Baumer, Allied Vision, FLIR, and others.
DeepInspect requires fewer than 200 good images for model training, significantly reducing setup time for AI defect detection.
No. DeepInspect uses unsupervised AI, eliminating the need for NG images in training.
Model training is completed in less than 45 minutes, allowing rapid deployment of vision inspection
A line demo for DeepInspect’s inspection system can be conducted within a day